If you think of an ‘elect-rode’ as being an electric rod, you are close to the truth. Another clue comes from the origin of the name. ‘Rhode’ means ‘a pathway’ in Greek. Hence electrodes are conductors through which electricity enters or leaves a substance or an object.
CHINA WELDING ROD 6013 WELDING ELECTRODES AWS E6013
The Role of Electrodes in the Transfer of Energy
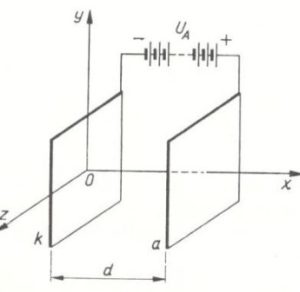
In the case of lead-acid batteries, electrodes transfer energy to and from the electrolyte in order to power the polarized device to which they connect. This energy leaves the battery via the negatively charged anode, and passes through the device. Then it returns via the positively charged cathode thereby reducing the power stored, through a process called reduction.
With rechargeable batteries electrodes can change roles. We call rechargeable batteries ‘secondary cells’ and non-rechargeable ones ‘primary cells’. To help you remember this, rechargables have secondary lives, but primaries only one. There are many different types of primary and secondary batteries in the global battery market.
How Electrodes Function in Lead-Acid Batteries
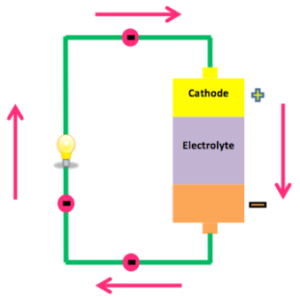
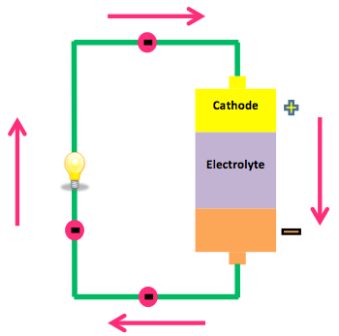
All batteries have cathodes and anodes, and an electrolyte which separates the two. This is the source of the chemical reaction that batteries convert to electricity. Oxidization causes an accumulation of electrons on the anode. This restless energy want to go somewhere, but the electron-less cathode is on the far side of the insulating electrolyte.
If we connect the two electrodes via a suitably rated device that controls the flow, some of the electrons pass through it to find their new home in the cathode. We can measure the voltage and current with a multimeter. We can also use this wonderful source of energy to power an almost infinite number of devices.