Tag/welding electrode e318
-
 E6013 Welding Electrode Rods for carbon steel
E6013 Welding Electrode Rods for carbon steel -
 Best Arc J422 6013 Welding Rod Electrodo E6013 3/32
Best Arc J422 6013 Welding Rod Electrodo E6013 3/32 -
 AWS E6013 Welding Rod Soldadura Electrodo J421
AWS E6013 Welding Rod Soldadura Electrodo J421 -
 China welding rod 6013 welding electrodes aws e6013
China welding rod 6013 welding electrodes aws e6013 -
 2.0mm (5/64”) E6013 Welding rod Electrodes Manufacture
2.0mm (5/64”) E6013 Welding rod Electrodes Manufacture -
 Welding Rods 6010 for welding carbon steel tubes
Welding Rods 6010 for welding carbon steel tubes -
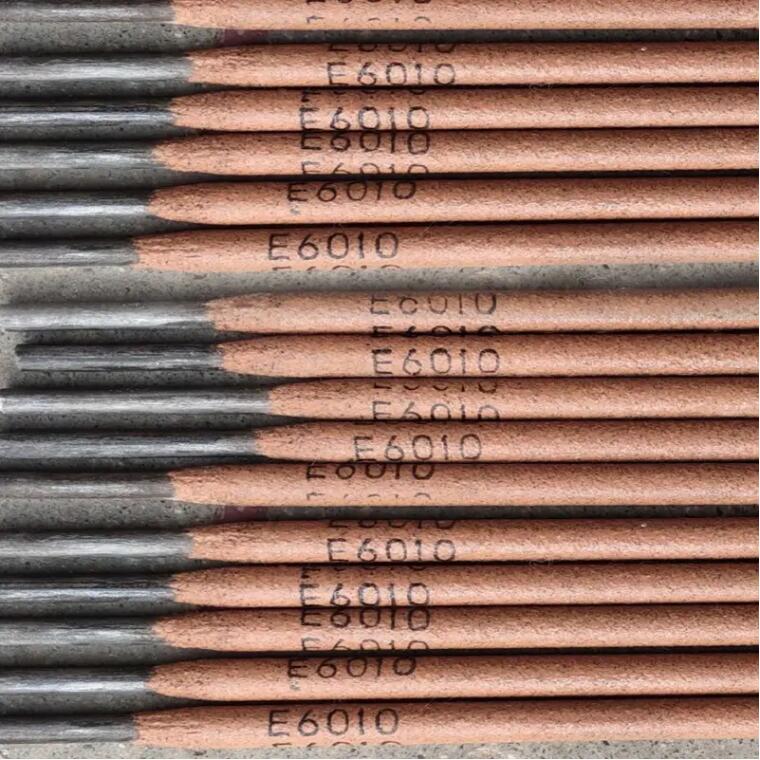 Cellulose Welding Rod E6010 for Carbon Steel Pipes
Cellulose Welding Rod E6010 for Carbon Steel Pipes -
 Pipeline welding rod E6010 cellulose welding rod
Pipeline welding rod E6010 cellulose welding rod -
 AWS E6010 Welding Electrodes Rods for Carbon Steel Pipes
AWS E6010 Welding Electrodes Rods for Carbon Steel Pipes -
 AWS E6010 Welding Electrodes Sticks
AWS E6010 Welding Electrodes Sticks
Produtct Title
welding electrode e318-
Welding Rods Electrodes AWS E6011 ELETRODO

-
Cellulose vertical downward Welding Electrode E6011

-
Electrodo 6011 Welding Rod E6011 Manufacture

-
Best Arc Welding Rods Cellulose AWS 6011 Electrode

-
China Welding Rods AWS Welding Electrode 6011

-
Welding electrode Rod J506 AWS E7016

-
Wholesale welding electrode e7016 welding rods

-
AWS A5.1 E7016 Carbon Steel Welding Electrode
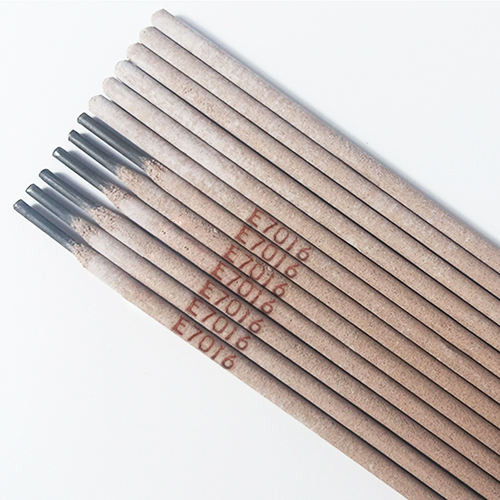
-
Customized E7016 Welding Rods Electrodes

-
E7016 welding electrodes rod manufacturers

-
Z408 Pure Nickel Cast Iron Electrode AWS ENiFe-CI

-
Co2 Gas Shielded Copper plating Micro wire

-
Copper Coated Welding Wire for carbon steel

-
Metal Spool K300 Welding Wire

Related News
-
2025-01-14There are several factors to consider in welding rod selectionBase metal properties. You need to know what type metal, what thickness metal you will used on, Base metal thickness, shape and joint fit-up.
-
2025-01-14Welding Electrodes ProductionWelding Electrodes Production Factory: Meeting the Demand for Quality and Reliable Welding RodsFor over 20 years, our welding electrodes production factory has been meeting the needs of industries that rely on strong and durable welding rods. Our commitment to quality and precision has made us a trusted name in the welding industry, and our team of professionals ensures that every welding electrode that leaves our factory meets the highest standards of excellence.
-
2023-10-13Welding wire new technologyAs one of the largest manufacturers of welding wire, our company is committed to producing high-quality products that meet the evolving needs of the welding industry.
-
2023-11-15About the welding consumables productionGas shielded welding wire is an essential component in the welding industry, providing a clean an
-
2024-01-31electrodes-What is an electrode?In general, an electrode is an electrical conductor which makes contact with a non-metallic part of a circuit. In a battery, the electrodes connect the battery terminals to the electrolyte. The electrode at the positive terminal is known as the cathode and the electrode at the negative terminal is known as the anode. Each electron is itself polarized, so that where they contact the electrolyte, the cathode is negatively charged and the anode is positively charged.
-
2024-11-22electrodes-What is Electrode?Electrodes can be defined as conductors that are used to make electrical contact with a non-metallic part of the circuit. The term was first coined by William Whewell and derived from Greek words Elektron, which means “amber” and hodos which translates to “a way.”An earlier version of an electrode was the electrophore which was used to study static electricity. It was invented by Johan Wilcke. To help you understand the concept in simple terms, an electrode is a point where the current enters and leaves the electrolyte. Notably, an electrode does not necessarily have to be metals.
-
2024-11-22electrodes-What is an Electrode?In an electrochemical cell, reduction and oxidation reactions take place at the electrodes. The electrode at which reduction takes places is called the cathode. Oxidation takes place at the anode.Whether an electrode operates as a cathode or anode depends on the direction the cell is operating in.If a cell is switched from operating galvanically (i.e. outputting energy like a battery) to electrolysis (energy is input to the cell) then its cathode will become its anode and vice versa.
-
2024-11-22electrodes-What is an electrode?Electricity is essential to life and much of what happens throughout the cosmos. But this flow of electrons does not just occur anywhere and everywhere. It flows as a current and along paths. Metals offer a great path. That’s why scientists refer to metals as conductors: They conduct electricity. But to move electricity through non-metallic materials, scientists need an electrolyte. This is a substance that contains ions — charged particles — that allow the current to flow.
-
2024-11-22electrodes-What are the mechanics of an electrode?An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it is known as the cathode and when the current enters it is known as the anode. Electrodes are vital components of electrochemical cells. They transport produced electrons from one half-cell to another, which produce an electrical charge. This charge is based off a standard electrode system (SHE) with a reference potential of 0 volts and serves as a medium for any cell potential calculation.
-
2024-01-31electrodes-what is an electrodeAn electrode is a conductor that is used to make contact with a nonmetallic part of a circuit.[1] Electrodes are commonly used in electrochemical cells (see Figure 1), semiconductors like diodes, and in medical devices. The electrode is the place where electron transfer occurs.An electrode is classified as either a cathode or an anode depending on the type of chemical reaction that occurs. If an oxidation reaction occurs at an electrode (oxidation being the loss of electrons), then the electrode is classified as an anode. If a reduction reaction occurs at an electrode (reduction being the gain of electrons), then the electrode is classified as a cathode.[2] Conventional current, in something like a discharging battery, flows into a device through its anode and leaves the device through the cathode.[3]
-
2024-11-22electrodes-What is an Electrode?In transcranial Direct Current Stimulation and brain stimulation in general the word “electrode” comes up a lot! As in “anode electrode”, or “cathode electrode”, or “the electrode was placed over this brain region”. So what is it?According to the definitive “Transcranial electrical stimulation nomenclature” published in Brain Stimulation we need to think about “Electrode Assembly” and “Electrode”. A lot of times when we say electrode in tDCS we mean electrode assembly. In fact in tDCS we never say electrode assembly. So below is a rather detailed and precise look at things.Don’t have the time to read it all now? Here are the takeaways: Electrode design is among the most important things in a good tDCS device. In practice, in tDCS, the electrode refers to the part of the device that is placed on the head and carrier the current from the tDCS device into the head. Any non-conducting material that is bound to the conductive components (like a rubber backing) is also considered part of the electrode. But headgear used to hold the electrode in place and the cables are not considered part of the electrode. And electrode must have a metal or conductive rubber in it. And electrode must also have an electrolyte, which is anything semi-fluid with salt in it. The electrolyte, not the metal or rubber, should touch the skin. In chemistry, the electrode is much more specific and refer to the interface between the metal or conductive rubber and the electrolyte.
-
2024-11-22electrodes-What is an electrode?According to wiki, an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte or a vacuum). Examples of electrodes are the cathode and anode.Given this definition, does this mean that every electric component that has electrodes (i.e. anode or cathode), is a nonmetallic part? And what exactly is the use of an electrode? Is it just used to indicate polarity? Are there other types besides anode or cathode? Can I use electrode as a synonym for pole? I hope someone can explain this without getting too technical.Given this definition, does this mean that every electric component that has electrodes (i.e. anode or cathode), is a nonmetallic part?Under that definition, in order to have electrodes a component must have nonmetallic parts. But it can't be entirely non-metallic since the electrodes are part of the component and they are metal. For example, a vacuum tube is generally made from metal, glass, and empty space (vacuum). It has both metallic and non-metallic components.
-
2024-11-22electrodes-What is an Electrode?An electrode is defined as a point where current enters and leaves an electrolyte. When current leaves an electrode, it is known as a cathode, and when it enters it is known as an anode. Electrodes transport electrons to one half cell to another producing an electrical charge. The charge is measured with the standard electrode system (SHE) with a reference potential of 0 volts which serves as a medium for any cell potential calculation. Electrons are vital components of electrochemical cells.
-
2024-02-23electrodes-What is an Electrode?An electrode is defined as a point where current enters and leaves an electrolyte. When current leaves an electrode, it is known as a cathode, and when it enters it is known as an anode. Electrodes transport electrons to one half cell to another producing an electrical charge. The charge is measured with the standard electrode system (SHE) with a reference potential of 0 volts which serves as a medium for any cell potential calculation. Electrons are vital components of electrochemical cells.
-
2024-02-23electrodes-What is an Electrode?An electrode is a conductor that comes into contact with the earth. An electrode is a conductor that establishes electrical contact with a nonmetallic component of a circuit. In electrochemical batteries, semiconductors like diodes, and medical equipment, electrodes are frequently used. Electron transfer occurs at the electrode.An electrode is categorized as either a cathode or an anode depending on the type of chemical reaction that occurs. It is referred to as an anode if it goes through the oxidation process (the loss of the electron).It is categorized as a cathode if a reduction process occurs on it. Similar to a discharged battery, a gadget that uses conventional current has an anode and a cathode.Between active electrodes and inactive electrodes, there are differences. Because it aids in the oxidation-reduction process, a magnesium electrode, for instance, is frequently regarded as an active electrode.A platinum electrode is frequently inactive because it does not participate in the oxidation-reduction process. Chemically inactive, an inert electrode serves only to permit current to flow through the electrochemical cell.
-
2024-02-23electrodes-What is an Electrode?In transcranial Direct Current Stimulation and brain stimulation in general the word “electrode” comes up a lot! As in “anode electrode”, or “cathode electrode”, or “the electrode was placed over this brain region”. So what is it?According to the definitive “Transcranial electrical stimulation nomenclature” published in Brain Stimulation we need to think about “Electrode Assembly” and “Electrode”. A lot of times when we say electrode in tDCS we mean electrode assembly. In fact in tDCS we never say electrode assembly. So below is a rather detailed and precise look at things.
-
2024-02-23electrodes-What is an Electrode?A conductor that makes contact with a nonmetallic component of a circuit is referred to as an electrode. Electrodes are widely employed in electrochemical cells, semiconductors such as diodes, and medical devices. The electrode is the site of electron transport.Depending on the sort of chemical process that happens, an electrode is classed as a cathode or an anode. If an electrode undergoes an oxidation process (the loss of the electron), the electrode is defined as an anode. If an electrode undergoes a reduction reaction, the electrode is classed as a cathode. Conventional current enters a device through its anode and exits through its cathode in something like a discharged battery.There is a variation between active electrodes and inert electrodes. A magnesium electrode, for example, is often an active electrode because it contributes to the oxidation-reduction process. Since it does not engage in the oxidation-reduction process, a platinum electrode is often inert. An inert electrode is chemically inert and exists merely to allow current to pass through the electrochemical cell.
-
2024-02-23welding rod-What Are Electrodes & What Do They Do?In the case of lead-acid batteries, electrodes transfer energy to and from the electrolyte in order to power the polarized device to which they connect. This energy leaves the battery via the negatively charged anode, and passes through the device. Then it returns via the positively charged cathode thereby reducing the power stored, through a process called reduction.With rechargeable batteries electrodes can change roles. We call rechargeable batteries ‘secondary cells’ and non-rechargeable ones ‘primary cells’. To help you remember this, rechargables have secondary lives, but primaries only one. There are many different types of primary and secondary batteries in the global battery market.
-
2024-02-23welding wire-HOW TO MAKE GOOD CHOICES FOR ELECTRODEAn electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are commonly used in electrochemical cells, semiconductors like diodes, and in medical devices. An electrode is classified as either a cathode or an anode depending on if current is flowing into or out of the electrode. Conventional current flows into a device through its anode and leaves the device through the cathode. An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). The word was coined by William Whewell at the request of the scientist Michael Faraday from two Greek words: elektron, meaning amber (from which the word electricity is derived), and hodos, a way.An electrode in an electrochemical cell is referred to as either an anode or a cathode. The anode is now defined as the electrode at which electrons leave the cell and oxidation occurs (indicated by a minus symbol, "−"), and the cathode as the electrode at which electrons enter the cell and reduction occurs (indicated by a plus symbol, "+"). Each electrode may become either the anode or the cathode depending on the direction of current through the cell. A bipolar electrode is an electrode that functions as the anode of one cell and the cathode of another cell.
-
2024-02-23electrodes-How to Make an ElectrodeInclude electrodes as part of a secondary cell, such as a rechargeable battery. The manufacture of electrodes in secondary cells is similar to that of electrodes in primary cells. However, the electrochemical reaction is reversible in a secondary cell. Therefore, the electrode that is the anode while the battery is charging will become the cathode while the battery is discharging. Similarly, the electrode that is the cathode while the battery is charging will become the anode while the battery is discharging. For example, in an nickel-cadmium battery, the cathode contains cadmium and the anode contains nickel. The battery produces an electrical current when the cadmium flows to the anode and nickel flows to the cathode. Applying an electrical current causes the nickel and cadmium to flow back to their original electrodes, thus recharging the battery.
Related Search
- welding rod
- welding electrode
- electrodes welding
- welding stick
- welding wire
- welding rod electrodes
- welding electrode rod
- electrode welding rod
- 6013 welding rod
- welding rod 2.5mm
- carbon steel welding rod
- stainless steel welding rod
- cast iron welding rod
- aluminum welding rod
- welding rods 308
- enife-ci welding rod
- welding rods 6010
- welding rod manufacturers
- metal welding rod
- welding rod j422
- 7018 welding rods electrodes
- 6010 welding rods
- welding rod 3/32
- welding rod e7018
- e7016 welding rod
- steel welding rod
- e7018 welding rod
- welding rods 1/16
- e7018 welding electrode
- welding electrode e7016
